The Baldwin Effect and Adult Hippocampal Neurogenesis
An Evolutionary Explanation for Lifelong Adult Hippocampal Neurogenesis in Humans and the Evolution of the Mental Immune System
"Adult hippocampal neurogenesis does not exist!" This was the retort I received after my lecture on the formation of new nerve cells in adults and their importance for mental health. The remark, quickly followed by the critic's reference to her scientific credentials, did not surprise me. Since its discovery, researchers who have demonstrated so-called "adult hippocampal neurogenesis" (AHN), study it, or work to educate the public about it have faced an unexpectedly frosty reception from some neuroscientists.
However, this moment made me reflect in a different way: While I have already explored this topic in depth in my comprehensive book publications, I realized the need for a concise article that clearly presents the essential arguments in an accessible way for everyone.
In this article, you will discover whether AHN is merely an evolutionary remnant without function, as critics suggest, or whether it actually plays a pivotal role in maintaining mental flexibility and adaptability well into old age. Even if you have read my books, you might find some fascinating new perspectives here that I have not previously addressed.
Dear reader,
This article was available exclusively to my paid subscribers for a couple of weeks before being released to all readers, as is customary on my substack. The information is too important to limit, but as a gesture of thanks, I am giving my paid subscribers early access.
Michael Nehls
New Nerve Cells for New Behaviors – Even in Old Age
The discovery of adult hippocampal neurogenesis (AHN) by Hungarian-American neurobiologist Joseph Altman (1925–2016) in 1962 laid the groundwork for our ever-expanding understanding of its existence and function.[1] Altman and his then-student Gopal D. hypothesized that the newly formed nerve cells they observed might serve as the "modulating and plastic elements" in an animal's response to its "diverse external environment" – in other words, that they facilitate learning and behavioral adaptation in an ever-changing world.[2]
As an independent researcher at MIT (Massachusetts Institute of Technology), Altman's findings were, by his own account, largely ignored in favor of those by Yugoslav-American neuroscientist Paško Rakić, who argued that neurogenesis is confined to prenatal development.[3] "For many years, the concept of adult neurogenesis was rejected and marginalized by influential figures in the neurosciences," a 2020 review of the history of adult neurogenesis reports, "sometimes even going so far as to censor studies and launch personal attacks against its proponents."
By the late 1990s, a paradigm shift occurred – but the harsh criticism remained: what must not be, cannot be.
The fact that the brain can generate new nerve cells in adulthood was rediscovered in 1999 by Elizabeth Gould and quickly became one of the hottest research topics in neuroscience.[4] To this day, there are both further confirmatory studies on the subject and ongoing critical voices. Rakić, for instance, continues to adhere to the view that, despite all evidence to the contrary, AHN has no function at all. If it exists in humans, he argues, it is at most a rudimentary evolutionary remnant of a tissue repair mechanism found in simpler brains. According to Rakić, organisms with complex brains, once neuronal development is complete, are like a neurological appendix – useless or at best nearly useless.[5]
The idea that AHN’s function could involve repair, as it does in simpler brains, is ruled out upon closer examination. This is because nerve cells in complex brains form tens of thousands of connections with other nerve cells. Since these connections are based on the individual’s experiences, replacing individual nerve cells would inevitably cause the loss of their specific functions and the memories they store.
However, Rakić tends to favor the view that AHN does not exist in humans, not even in rudimentary form. His reasoning is that the human brain prioritizes stability over plasticity to carry out its functions. In his words: “A stable population of neurons in primates, including humans, may be important for the continuity of learning and memory throughout the lifespan.” A 2008 paper similarly argues that this reduction in adult neurogenesis “corresponds to the transition from unpredictable to predictable behavior during adolescence, which is characteristic of mammalian behavior after reaching reproductive maturity.”[6]
Evolutionary Aspects of Adult Hippocampal Neurogenesis
The most significant conceptual argument against the existence of AHN in humans – namely, that established neuronal networks require stable connections and that adding new nerve cells would disrupt the stability of these networks and, consequently, cognitive abilities – has been refuted by both computer network simulations[7] and behavioral studies[8].
Rakić's restrictive perspective is fundamentally challenged by the fact that higher mammals, especially, not only retain the ability to learn new behaviors well into old age but also appear to rely on this capacity. It also directly contradicts the scientific concept of “grandmother evolution,” which posits an evolutionary basis for adult women becoming grandmothers who, after the end of their own reproductive activity, live for decades to support their grandchildren and thereby enhance their survival prospects. In essence, this concept highlights the evolutionary advantage of transgenerational reproductive experience, which underpins the exceptional longevity of humans.[9]
The German physician Gerd Kempermann, one of the world's leading researchers on AHN, along with many colleagues, even hypothesizes that AHN evolved specifically in mammals. They argue that AHN “bears little resemblance to the more diffuse neurogenesis found in analogous structures in non-mammals.”[10] Accordingly, they propose that AHN represents “an advanced solution for a specific network situation, endowing the hippocampus with additional specialized functions – including in humans.”
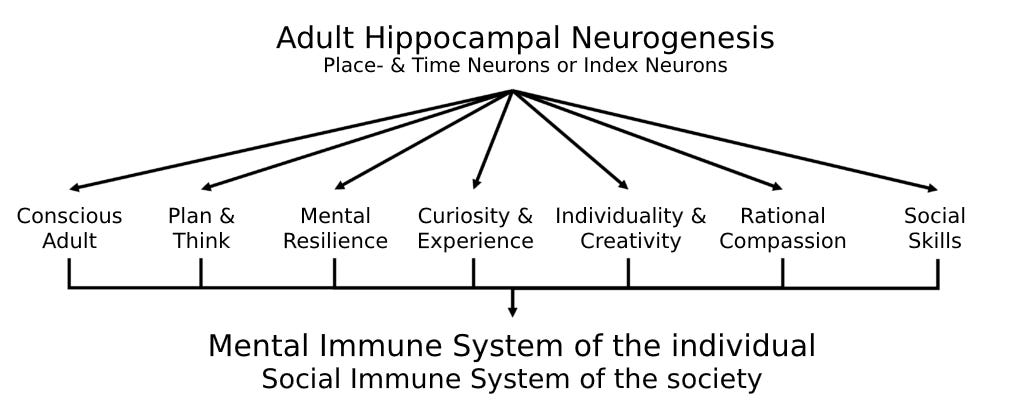
I describe this exceptional capacity for learning and adaptation to a new “network situation,” as it is termed here, particularly in humans, as the full functional scope of the mental immune system (see figure).
Functional Arguments for the Existence of Adult Hippocampal Neurogenesis
The Problem of Quantification and the Importance of Quality
A fundamental function of these newly forming hippocampal nerve cells, acting as place and time memory, is to anchor all autobiographical memory content within the four-dimensional space-time continuum of our existence. By creating a specific index for each experience, thought, plan, dream, and hope, they allow us to retrieve memories in a targeted and precise manner. These neurons form the foundation for the development of self-awareness, the ability to reflect, and the creative planning and visualization of future tasks and their execution.
These place and time neurons, which I refer to as index neurons due to their functional combinatorial role in reconstructing our memories, enable us to differentiate between the past, our current experiences, and our future plans, all of which are also stored in the hippocampus.[11] This capacity allows us to discern what we have personally experienced, what we have learned from others, and what we have merely imagined. Thus, they are instrumental in helping individuals distinguish between their own and others' experiences, thereby fostering the emergence of an autonomous self.
Without the functional properties of these index neurons, what neurologists (and increasingly AI researchers) describe as catastrophic interference would occur: daydreams and real experiences would collide and become indistinguishable. For individuals suffering from schizophrenia – a condition causally linked to developmental or maturation disorders of the hippocampus – this may represent a central issue underlying their illness.[13]
Even though science has not yet reached a definitive consensus (as of autumn 2024) on how many new nerve cells are formed in the hippocampus each day – estimates vary from several thousand to perhaps only a few hundred, depending on the methodology and the ‘human material’ used – they play a crucial, and in my opinion, even central role in the functioning of our mental immune system.
As early as 2018, Kempermann and colleagues resolved the discrepancies between different study results—namely, the very high AHN production rates observed into old age in some studies versus the rather low rates reported in others—and detailed their findings in a review article. They emphasized that regardless of any definitive figure future studies may establish as age-dependent normative values in optimally healthy individuals, it is not the quantity (i.e., the number) but the quality of the newly formed nerve cells that is decisive.
In contrast to the rest of the hippocampal network, they write, ‘synaptic plasticity [learning and adaptability] in the dentate gyrus [the entry region of the hippocampus, where neurogenesis occurs throughout life] is concentrated on a defined, functionally naive [fresh and as yet unused] subgroup of (new) neurons.’ This unique mechanism of focused plasticity distinguishes this neuronal network from all others studied so far. They conclude that ‘the number of new cells necessary for a functional effect is very small.’
The findings of a recent study employing state-of-the-art methods, published in October 2024, point to a low but persistent neurogenesis in the hippocampus throughout life. According to the researchers, this confirms ‘the existence of a local reserve of plasticity in the hippocampus of the adult human.’
The significance of adult hippocampal neurogenesis (AHN) well into old age is strikingly demonstrated by another recent study—with one critical prerequisite: AHN can only be detected in healthy individuals. The study by Boldrini et al. (2018) reveals that AHN persists in healthy humans at least into their eighth decade of life, offering the potential to sustain cognitive and emotional functions even in advanced age.
Notably, the study suggests that ongoing neurogenesis in the hippocampus may underpin human-specific cognitive abilities throughout the entire lifespan and that a decline in these processes is linked to reduced cognitive-emotional resilience. The findings are based on a comprehensive analysis of whole post-mortem hippocampi from individuals aged 14 to 79 who were healthy during their lifetime. Immature and maturing neurons were observed only in those without underlying neurological conditions. This underscores the strong association between AHN and overall individual health.
The study not only provides crucial evidence for the existence of AHN but also highlights that maintaining a healthy lifestyle is essential for preserving hippocampal neurogenesis, thereby safeguarding cognitive and emotional adaptability well into old age.
The absence or scarcity of AHN in the brains of unhealthy individuals provides evidence that lifestyle has a profound impact on mental health
A report published in late February 2024, titled ‘Impact of Adult Neurogenesis on Emotional Functions: In Mice and Humans,’ summarizes that ‘there is growing anatomical, biochemical, and genomic evidence for the presence of immature neurons in the hippocampus of healthy individuals throughout life.’[17]
Once again, the keyword is ‘healthy.’ Indeed, a Spanish study identified thousands of immature and maturing neurons in the hippocampi of healthy individuals across a wide age range (even up to 90 years!). Methodologically, this study left no unanswered questions. As the authors wrote in a subsequent review: ‘We provide a detailed description of the methods employed in our laboratory to unequivocally identify a population of immature neurons in the human hippocampus up to the tenth decade of life.
The criteria used to refine and develop the current protocol included obtaining high-quality postmortem human samples under strictly controlled conditions for immunohistochemical (IHC) studies, optimizing tissue processing and histological techniques, establishing criteria for reliable validation of antibody signals, and conducting unbiased stereological cell counts.’[19]
The importance of tissue material from healthy deceased individuals is further underscored by findings showing that the number and maturation of these new nerve cells decrease drastically over the course of Alzheimer’s disease, which I refer to as hippocampal dementia due to its site of origin. This was confirmed by an independent study in Chicago involving deceased individuals with an average age of 90 years: the more deficient the AHN, the more advanced the Alzheimer’s disease. According to the authors of the Spanish study, their findings provide ‘evidence for impaired neurogenesis as a potentially relevant mechanism underlying memory deficits in Alzheimer’s disease, which could be amenable to new therapeutic strategies.’
This aligns closely with my systems theory of Alzheimer’s disease, published in 2016, in which I was among the first scientists worldwide to identify disrupted AHN—caused by an unnatural way of life—as the core issue and a key focus for practical approaches to preventing Alzheimer’s dementia.[23] This perspective is now supported by many other researchers. Based on these latest findings, gerontologists at the influential Harvard University in Boston are now asking: ‘Is Alzheimer’s a neurogenesis disorder?’[24]
Restoring AHN and the Resulting Health Benefits
It has now been demonstrated that activating AHN can literally allow us to "outrun" Alzheimer's. A growing hippocampus keeps us young—at least mentally. So, as I put it in my book The Exhausted Brain, it is never too late for an "active childhood."[25]
In a movement study examining hippocampal volume and aspects of the mental immune system, researchers selected 120 seniors with an average age of around 70 who had age-appropriate mental health. (If we consider "normal" to describe these participants—who had not walked more than 30 minutes per week in the six months prior to the study—it is unfortunately also "normal" that this description applies to most older residents of social housing.)[26]
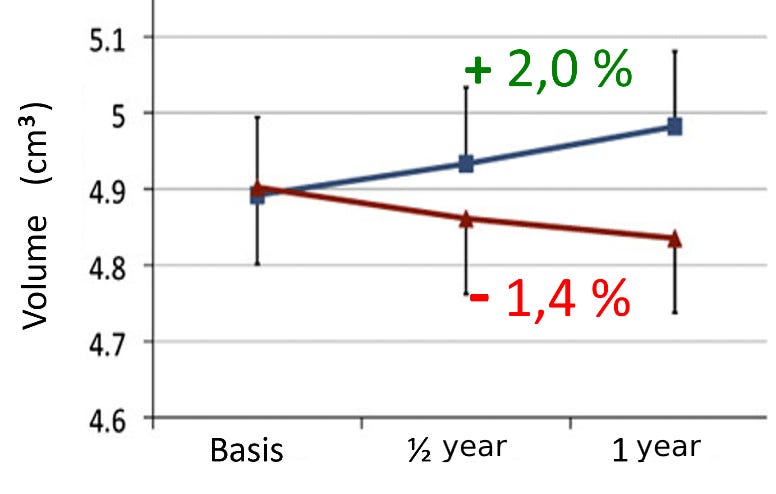
Initially, participants were randomly divided into two equal groups. One group was instructed to walk for approximately 40 minutes every day, while the other group performed seated stretching exercises for the same duration. Over the course of a year, participants either engaged in walking or stretching. Brain imaging was conducted volumetrically on all participants at the beginning, midpoint, and end of the study—with clear results: among the walkers, the hippocampus grew by an average of about two percent over the year (see graph), with the increase in volume being more pronounced in participants who became fitter through physical training during the study.
Similar improvements were observed in autobiographical memory performance and psychological resilience, both of which are fundamental functions of the mental immune system. The key term is ‘psychological resilience,’ which provides protection against Alzheimer’s disease but is ultimately a function of the mental immune system and active AHN.[27]
In contrast, the hippocampal volume in the stretching group decreased by approximately 1.4 percent (see graph), along with a decline in mental performance.
Clear findings from a meta-study (which combines and analyzes multiple studies) further support this correlation. Scientists from the Department of Psychology at the University of Pittsburgh concluded that ‘higher cardiorespiratory fitness levels are consistently associated with greater grey matter volume in the prefrontal cortex and hippocampus, and less consistently in other regions.’[28]
This holds true not only for older age groups but also for young people. This is further illustrated by the findings of an international study, which concluded that ‘higher cardiorespiratory fitness is important for enhancing the health of children and adolescents as well as improving their academic performance.’[29]
The Baldwin Effect: Innate Behavioral Flexibility and Learning Ability into Old Age
Since AHN has been shown to persist into old age in humans, the question remains: Is it merely a vestigial remnant from the evolution of originally more primitive brains, where new nerve cells primarily served a repair function in the adult organism? Or is AHN, as Kempermann and colleagues (and I) propose, an acquired higher function that requires the daily generation of new cells as needed?
One potential answer lies in the so-called Baldwin Effect, proposed by a group of neuroscientists from Bordeaux, France, as a conceptual compromise between these two hypotheses.[30] Named after the American philosopher and psychologist James Mark Baldwin (1861–1934), the Baldwin Effect describes a mechanism of ‘ontogenetic adaptation’: a behavior acquired during individual development (ontogenesis) is initially transmitted to the next generation through imitation. In doing so, it permanently alters the social environment for subsequent generations, eventually favoring the optimization of learning effects (or learning abilities) through genetically anchored traits.
In other words, new social or cultural developments can drive genetic adaptations, which in turn enable improved responses to those same social or cultural circumstances. The Baldwin Effect differs from the Lamarck Effect, named after Jean-Baptiste de Lamarck (1744–1829), in which behaviorally acquired traits are epigenetically inherited for a limited number of generations.[31]
However, it is also not pure Darwinism, as discovered by Charles Robert Darwin (1809–1882), where random but advantageous mutations are selected through improved reproductive success.
When applied to the question of AHN’s existence, the Baldwin Effect presents an evolutionary scenario: individuals with a highly functional mental immune system, rooted in the still rudimentary AHN, gained a social advantage. With the growing complexity of adaptation required to meet the newly created social and cultural circumstances, this new functionality became increasingly significant—after all, it continues to play a critical role in ensuring human survival to this day.
This represents a combination of both hypotheses: the idea that AHN initially served as a vestige of the repair capacity of simpler brains but was repurposed for novel and successful functions due to social demands, thereby conferring a selective advantage. The persistence of AHN in more complex brains thus introduces a new functionality—adaptability—whose content (i.e., ‘How should I behave?’) is not inherited (it does not constitute instinctive behavior) but can instead be individually learned or newly developed via AHN. This adaptability even enables us to plan and execute entirely new and complex behaviors—in other words, precisely what I define as the mental immune system.
Since the Baldwin Effect enables individuals to learn or develop new behaviors that are essential for survival or highly beneficial, yet cannot be inherited as automatisms or instincts, with the help of a permanently active AHN, the French neuroscientists argue that ‘the ability of adults to adapt their behavior is based on AHN.’ They further state:
‘In this review, we argue that [AHN] is indeed subject to a selection effect and serves an evolutionary function. However, this function must be understood in a specific context [...] i.e., an evolutionary situation where a particular function does not disappear but also cannot become genetically hard-wired, thereby relying on individual adaptation that recurs in every generation.
To understand this perspective, it is important to recognize that [AHN] likely served a variety of functions at some stage in evolution. Its primary function was most probably tissue repair in simpler brains, where neurons with relatively few connections are easier to replace.[32]
The more complex a brain becomes, the harder it is to repair. Thus, there may have been a point in evolution where the advantages of complexity outweighed those of reparability in specific environments. At that stage, [AHN] likely lost its tissue repair function. This does not mean it lost all functionality. In fact, it may have regressed in most parts of the brain except in certain specialized regions, where its maintenance at a modest level enhanced the fitness of the individual despite the costs associated with sustaining neurogenesis. This is the case in the dentate gyrus [DG] of the hippocampus.’
Summary
The emergence and utilization of the small yet highly functional population of new nerve cells in the dentate gyrus of the human hippocampus through AHN is far from a mere scientific side topic with purely theoretical relevance—on the contrary, this function is fundamentally important to all our lives and our modifiable health.
In my view, AHN, as the foundation or neuronal correlate of the mental immune system with its diverse and essential functions (see figure above), constitutes the core of what it means to be human. It is what enables us to become who we are and to value these qualities in ourselves. This includes the ability to seek and embrace new experiences with the help of our mental immune system, to be inquisitive, to plan and execute those plans, and ultimately, to secure a selective advantage through reflective experience and social competence—not only for ourselves but also, and more importantly, for our children, grandchildren, and the broader social community.
This aligns with the concept of the ‘grandmother’s evolution,’ which also allows grandfathers to remain mentally sharp and curious well into old age.
References
[1] Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962 Mar 30;135(3509):1127-8. doi: 10.1126/science.135.3509.1127. PMID: 13860748.
[2] Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965 Aug 28;207(5000):953-6. doi: 10.1038/207953a0. PMID: 5886931.
[3] Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425-7. doi: 10.1126/science.183.4123.425. PMID: 4203022.
[4] Gould E et al: Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999 Mar;2(3):260-5. doi: 10.1038/6365. PMID: 10195219.
[5] Rakic P. Limits of neurogenesis in primates. Science. 1985 Mar 1;227(4690):1054-6. doi: 10.1126/science.3975601. PMID: 3975601; Duque A, Arellano JI, Rakic P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol Psychiatry. 2022 Jan;27(1):377-382. doi: 10.1038/s41380-021-01314-8. Epub 2021 Oct 19. PMID: 34667259; PMCID: PMC8967762.
[6] Amrein I, Lipp HP. Adult hippocampal neurogenesis of mammals: evolution and life history. Biol Lett. 2009 Feb 23;5(1):141-4. doi: 10.1098/rsbl.2008.0511. PMID: 18957357; PMCID: PMC2657751.
[7] Deng W et al: Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009 Oct 28;29(43):13532-42. doi: 10.1523/JNEUROSCI.3362-09.2009. PMID: 19864566; PMCID: PMC2787190.
[8] Arruda-Carvalho M et al: Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011 Oct 19;31(42):15113-27. doi: 10.1523/JNEUROSCI.3432-11.2011. PMID: 22016545; PMCID: PMC6623574; Dupret D et al: Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008 Apr 9;3(4):e1959. doi: 10.1371/journal.pone.0001959. PMID: 18509506; PMCID: PMC2396793.
[9] Lahdenperä M et al: Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004 Mar 11;428(6979):178-81. doi: 10.1038/nature02367. PMID: 15014499; Hawkes K: Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons. Proc Natl Acad Sci U S A. 2010 May 11;107 Suppl 2(Suppl 2):8977-84. doi: 10.1073/pnas.0914627107. Epub 2010 May 5. PMID: 20445089; PMCID: PMC3024018.
[10] Kempermann G et al: Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 23:25-30, 2018, https://doi.org/10.1016/j.stem.2018.04.004.
[11] Montagrin A et al. The hippocampus dissociates present from past and future goals. Nat Commun 15, 4815 (2024). https://doi.org/10.1038/s41467-024-48648-9
[12] Freund J et al: Emergence of individuality in genetically identical mice. Science. 2013 May 10;340(6133):756-9. doi: 10.1126/science.1235294. PMID: 23661762; Zocher S et al: Early-life environmental enrichment generates persistent individualized behavior in mice. Sci Adv. 2020 Aug 26;6(35):eabb1478. doi: 10.1126/sciadv.abb1478. PMID: 32923634; PMCID: PMC7449688.
[13] DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010 May;58(6):884-93. doi: 10.1016/j.neuropharm.2009.12.013. Epub 2010 Jan 6. PMID: 20060007; PMCID: PMC2839019.
[14] Kempermann G et al: Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 23:25-30, 2018, https://doi.org/10.1016/j.stem.2018.04.004.
[15] Simard S, Rahimian R, Davoli MA, Théberge S, Matosin N, Turecki G, Nagy C, Mechawar N. Spatial transcriptomic analysis of adult hippocampal neurogenesis in the human brain. J Psychiatry Neurosci. 2024 Oct 16;49(5):E319-E333. doi: 10.1503/jpn.240026. PMID: 39414359; PMCID: PMC11495544.
[16] Boldrini , Maura et al: Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell, Volume 22, Issue 4, 589 – 599.e5. doi: 10.1016/j.stem.2018.03.015
[17] Alonso M et al: The impact of adult neurogenesis on affective functions: of mice and men. Mol Psychiatry. 2024 Aug;29(8):2527-2542. doi: 10.1038/s41380-024-02504-w. Epub 2024 Mar 18. PMID: 38499657; PMCID: PMC11412911.
[18] Moreno-Jiménez EP et al: Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019 Apr;25(4):554-560. doi: 10.1038/s41591-019-0375-9. Epub 2019 Mar 25. PMID: 30911133.
[19] Flor-García M et al: Unraveling human adult hippocampal neurogenesis. Nat Protoc. 2020 Feb;15(2):668-693. doi: 10.1038/s41596-019-0267-y. Epub 2020 Jan 8. PMID: 31915385.
[20] Steiner E, Tata M, Frisén J. A fresh look at adult neurogenesis. Nat Med. 2019 Apr;25(4):542-543. doi: 10.1038/s41591-019-0408-4. PMID: 30911138.
[21] Tobin MK et al: Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell. 2019 Jun 6;24(6):974-982.e3. doi: 10.1016/j.stem.2019.05.003. Epub 2019 May 23. PMID: 31130513; PMCID: PMC6608595.
[22] Moreno-Jiménez EP et al: Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019 Apr;25(4):554-560. doi: 10.1038/s41591-019-0375-9. Epub 2019 Mar 25. PMID: 30911133.
[23] Nehls M. Unified theory of Alzheimer’s disease (UTAD): implications for prevention and curative therapy. J Mol Psychiatry. 2016 Jul 15;4:3. doi: 10.1186/s40303-016-0018-8. PMID: 27429752; PMCID: PMC4947325.
[24] Choi SH, Tanzi RE. Is Alzheimer’s Disease a Neurogenesis Disorder? Cell Stem Cell. 2019 Jul 3;25(1):7-8. doi: 10.1016/j.stem.2019.06.001. PMID: 31271749.
[25] Nehls M: Das erschöpfte Gehirn: Der Ursprung unserer mentalen Energie – und warum sie schwindet – Willenskraft, Kreativität und Fokus zurückgewinnen. Heyne 2022
[26] Erickson KI et al: Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011, 108:3017-3022
[27] de Vries LE et al: The concept of resilience to Alzheimer’s Disease: current definitions and cellular and molecular mechanisms. Mol Neurodegener. 2024 Apr 8;19(1):33. doi: 10.1186/s13024-024-00719-7. PMID: 38589893; PMCID: PMC11003087.
[28] Erickson KI et al: Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014 Sep;35 Suppl 2:S20-8. doi: 10.1016/j.neurobiolaging.2014.03.034. Epub 2014 May 14. PMID: 24952993; PMCID: PMC4094356.
[29] Marques A et al: How does academic achievement relate to cardiorespiratory fitness, self-reported physical activity and objectively reported physical activity: a systematic review in children and adolescents aged 6-18 years. Br J Sports Med. 2018 Aug;52(16):1039. doi: 10.1136/bjsports-2016-097361. Epub 2017 Oct 14. PMID: 29032365.
[30] Abrous DN et al: A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology. Mol Psychiatry. 2022 Jan;27(1):383-402. doi: 10.1038/s41380-021-01172-4. Epub 2021 Jun 8. PMID: 34103674; PMCID: PMC8960398.
[31] https://www.mpg.de/4425822/epigenetische_veraenderungen
[32] Bonfanti L, Amrein I. Editorial: Adult Neurogenesis: Beyond Rats and Mice. Front Neurosci. 2018 Dec 4;12:904. doi: 10.3389/fnins.2018.00904. PMID: 30564088; PMCID: PMC6288481.






Is Mr. Nehls disclosing what supplements he is personally taking, in case we readers might want to cut to the chase and do the same? At least in regard to cardiovascular and brain health?